Research Projects
A. Cyclodextrin Inclusion
Complexes:
crystalline state and solution structures and properties
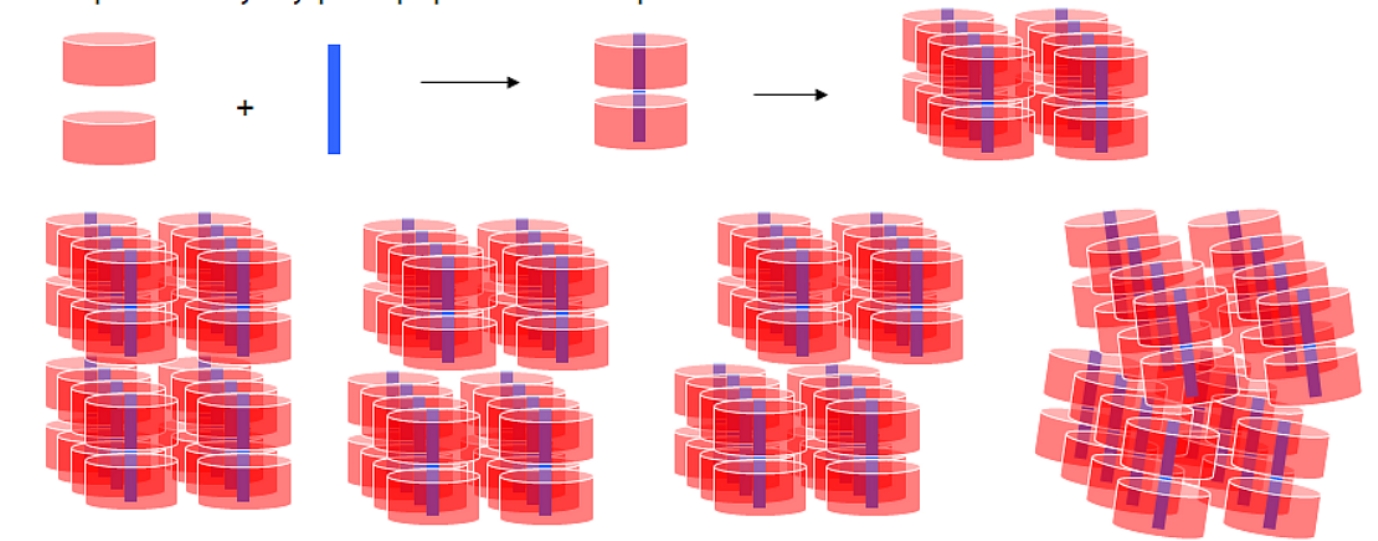
Figure 1.
Schematic view of the
βCD
(pink) dimer formation upon inclusion of a guest molecule (blue).
Organisation of the dimers into 2-D layers. Packing of the layers into
four distinct modes: (a) Channel
(CH), the guest in the cavity is shielded from the aqueous environment.
(b) Chessboard (CB), the
guest end-groups emerging from the primary sides of the dimer are
exposed to the aqueous environment. (c)
Intermediate
(IN)
a case in-between the CH and CB modes, adjacent layers are parallel, but
the dimer’s 7-fold axis forms an angle of about 20o with the
stacking axis. Consequently, dimers are far from exactly aligned, thus a
breaking of the channel is observed that leaves parts of the guests free
to interact with hydroxy groups of adjacent hosts, as well as with water
molecules. (d) Screw Channel (SC), where although the lateral
displacement between two consecutive
βCD
dimers along the channel is only 2.7Å,
as in the CH mode, the dimer’s 7-fold axis forms an approximately angle
of 10o with the stacking axis but the 2-D layers are related
by a 2-fold screw axis and they are not parallel therefore, the guests
interact with water molecules and hydroxy groups of adjacent hosts
channels.
References
1. D. Mentzafos, I. M. Mavridis,
G. Le Bas and G.Tsoucaris
“The
Crystal Structure of the 4-tert-Butylbenzyl Alkohol
β
-Cyclodextrin Complex.
Common Features in the
Geometry of the
β-cyclodextrin
Dimeric Complexes”
Acta Crystallogr.
1991,
B47,
746-757.
2. I.
M. Mavridis,
3.
I. M. Mavridis,
4.
A.
Rontoyanni, I. M. Mavridis,
5.
“Organization of Long
Aliphatic Monocarboxylic Acids in
β-Cyclodextrin
Channels.
Crystal
Structures of the Inclusion Complexes of Tridecanoic Acid and (Z)-tetradecenoic
Acid in
β-Cyclodextrin”,
S. Makedonopoulou,
I. M. Mavridis, K.
Yannakopoulou, J. Papaioannou,
J. Chem. Soc.
Chem. Commun.
1998, 2133-2134.
6.
S. Makedonopoulou, A. Tulinsky,
7.
Α.
Rontoyianni,
8.
S. Makedonopoulou, I. M.
Mavridis
“Structure of the Inclusion Complex of
β-Cyclodextrin
with 1,12-Dodecanodioic Acid using Synchrotron Radiation Data.
A Detailed
dimeric
β-Cyclodextrin
Structure”
Acta Crystallogr.
2000, B56, 322-331.
9.
S. Makedonopoulou, J. Papaioannou, I. Argyroglou,
10. S. Makedonopoulou, I. M.
Mavridis “The dimeric complex of beta
cyclodextrin with 1,14-tetradecanedioic acid.
Comparison with related
complexes”
Carbohydr. Res.
2001, 335, 213-220.
11. I. M. Mavridis
“Influence of the Guest on
the Packing of Dimeric
β-Cyclodextrin
Complexes”,
in Current
Challenges on Large Supramolecular Assemblies,
Ed. G.Tsoucaris NATO A
12.
“Molecular structures of
the inclusion complexes
β-Cyclodextrin/1,2-bis(4-aminophenyl)ethane
and
β-Cyclodextrin/4,4΄-diaminobiphenyl.
Packing of dimeric
β-Cyclodextrin
inclusion complexes”
13. S.
D. Chatziefthimiou, K. Yannakopoulou, I. M. Mavridis
“β-Cyclodextrin
trimers enclosing an unusual organization of guest:
The inclusion complex
β-Cyclodextrin/4-pyridinealdazine”
Cryst.
14. Κ.
Eliadou, K.
Yannakopoulou, A. Rontoyianni,
I. M. Mavridis
“NMR
Detection of Simultaneous Formation of [2]- and [3]Pseudorotaxanes in
Aqueous Solution between
α-Cyclodextrin
and Linear Aliphatic
α,ω-Aminoacids,
an
α,ω-Diamine
and an
α,ω-Diacid
of Similar Length, and Comparison with The Solid State Structures”
J. Org. Chem.
1999, 64, 6217-6226.
15. A. Tsortos, K. Yannakopoulou, K. Eliadou,
I.M. Mavridis and G.
Nounesis
“Partial Thermal Dethreading of
[3]pseudorotaxanes of
α-Cyclodextrin
with Linear Aliphatic
α,ω-Aminoacids
in Aqueous Solution” J. Phys. Chem.
2001, B105, 2664-2667.
16.
17. K.
Yannakopoulou and
I. M. Mavridis
“Threading of
long end-functionalised organic molecules into cyclodextrins: Structural
analysis in aqueous solution by NMR spectroscopy and in the solid state
by X-ray crystallography”
Current Org. Chem. 2004,
8, 25-34.
18. K. Yannakopoulou, and
19. K. Fotiadou, A. Thanassoulas, G. Nounesis, K. Yannakopoulou “Cooperative Heterodimer Formation Between Per-Guadinylated and Carboxylated or Phoshporylated Cyclodextrins in DMSO and DMSO-Water Studied by NMR Spectroscopy and Microcalorimetry” Supramol. Chem., 2011, 23, 493-500.
20.
A. Botsi, K. Yannakopoulou, E.
Hadjoudis and B. Perly
“NMR Differentiation of Enantiomeric (+)- and (-)-α-Pinene via
Complexation with Cyclodextrins in Water"
J. Chem.
Soc. Chem. Commun. 1993,
1085.
21.
A. Botsi, K. Yannakopoulou, E.
Hadjoudis
“Inclusion Complexes of Cyclomaltoheptaose
and its Methylated Derivatives with the Main Components of the
Pheromone of the Olive Fruit Fly" Carbohydr. Res.
1993,
241, 37.
22.
I.
M. Mavridis,
D. Mentzafos and H. Schenk
"Crystal Structure of the
Heptakis (2,3,6-tri-O-methyl)-β-cyclodextrin
Complex with Ethyldodecanoate Ester"
Carbohydr. Res.
1994,
253,
39-50.
23.
A. Botsi, K. Yannakopoulou, B. Perly and E. Hadjoudis
“Positive or Adverse Effects of Methylation on the Inclusion Behaviour
of Cyclodextrins. A Comparative
NMR Study Using Pheromone Constituents of the Olive Fruit Fly" J. Org. Chem.,
1995,
60, 4017.
24. A. Botsi, K. Yannakopoulou,
B. Perly and
25.
A. Botsi, K. Yannakopoulou, E. Hadjoudis, and J. Waite
“AM1 Calculations on Inclusion Complexes of Cyclomaltoheptaose with
1,7-Dioxaspiro[5.5]undecane and Nonanal, and Comparison with
Experimental Results" Carbohydr. Res.,
1996,
283, 1-16.
26.
K. Yannakopoulou, J. A.
Ripmeester,
27.
D. Mentzafos,
I. M. Mavridis “β
-Cyclodextrin (Z)-9-Dodecen-1-ol 2:1 Complex” Acta
Crystallogr. 1996,
C52, 1220-1223.
28. A. Kokkinou,
K. Yannakopoulou, I.M.
Mavridis, D. Mentzafos
“Inclusion Compounds of Plant Growth Regulators in Cyclodextrins. Part
II. Structure of the Complex of
β-Cyclodextrin
with
β-Naphthyloxyacetic
acid in solid state and in aqueous solution”
Carbohydr.Res.
2001,332, 85-94.
29.
V. Mazomenos,
I M. Mavridis
Eur. Patent No 92 401
709.8, 1992;
30. K.
Yannakopoulou, D. Mentzafos, I. M. Mavridis and K. Dandika
“Chiral Recognition of (R)-(_)-1,7-Dioxaspiro[5.5]undecane
by Hexakis(2,3,6-tri-O-methyl)-α-Cyclodextrin”
Angew. Chem. Int. Ed. Engl.
1996
35,
2480-2482.
31.
S.
Makedonopoulou, K. Yannakopoulou, D. Mentzafos, V. Lamzin,
A. Popov, and I. M. Mavridis
“Non-covalent interactions
in the crystallization of enantiomers of 1,7-dioxaspiro[5.5]undecane
(olive fly sex pheromone) by enantiospecific cyclodextrin hosts, hexakis(2,3,6-tri-O-methyl)-α-cyclodextrin
and heptakis(2,36-tri-O-methyl)-β-cyclodextrin”,
Acta Crystallogr.
2001,
B57, 399-409.
32. D.
Zouvelekis, K.
Yannakopoulou, A.
Antoniadou-Vyza, I.M. Mavridis
"The Self-Association of the Drug Acemetacin and its Intermolecular
Interactions and Stability with
β-Cyclodextrin
in Aqueous Solution. An NMR and HPLC Study", Carbohydr.
Res.,
2002,
337, 1387-1395.
33.
D.
Maffeo, L. Leondiadis,
34.
A.
Paulidou, D. Maffeo, K. Yannakopoulou, I M. Mavridis
“Crystal structure of the
inclusion complex of the antibacterial agent triclosan in
β-cyclodextrin
and NMR study of its molecular encapsulation in positively and
negatively charged cyclodextrins” Carbohydr.
Res.
2008 343, 2634-2640.
35. A. Paulidou, D. Maffeo, K. Yannakopoulou, I.M. Mavridis “Similar modes of inclusion in complexes of β-cyclodextrin with sulfonylurea hypoglycemic drugs” Cryst. Eng. Comm. 2010,12, 517-525.
36
